Kimberly Capone, Ph.D., Shifra Liba Klein, Ph.D., Frank Kirchner, M.S., Neena Tierney, Ph.D. Johnson & Johnson Consumer, Inc. Presented at the 76th Annual Society for Investigative Dermatology (SID) Meeting, Portland, OR, April 26-29, 2017
Purpose
Atopic Dermatitis (AD) lesions are often colonized by Staphylococcus aureus, and improvements in microbial diversity are reported to correlate with improvements in disease severity. In addition, follicular colonization by S. aureus has been implicated in exacerbating the sensations of itch in the AD cycle. Despite these advances, the function of the skin microbiota in the pathogenesis of AD remains poorly understood, particularly in different patient populations. Advances in our understanding of the AD-associated skin microbiome along the disease progression will facilitate deeper insights into the function of the skin microbiota in this complex ecosystem, and ultimately novel microbiome-based therapies. Therefore, we undertook to examine the changes in the skin microbiota, AD disease severity, and itch, during 14 days of treatment with two different topical lotions, including AVEENO® Eczema Therapy Daily Moisturizing Cream (specially formulated for use on AD patients) and a non-fragranced lotion. The 14-day treatment phase was followed by a 7-day regression phase to observe alterations in the skin microbiota with worsening disease
Clinical Design
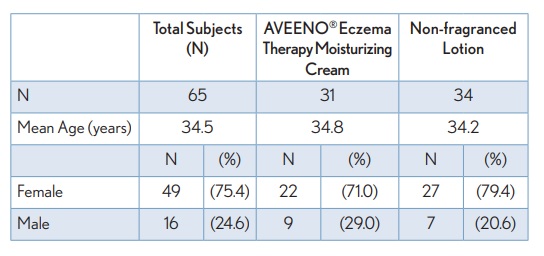
This multi-centre, randomized, controlled 14-day clinical usage study was conducted to associate the skin microbiota of AD patients with pruritus, or itch, during the use of 2 different topical lotions. The patient population consisted of female and male subjects aged 16 – 50 years with mild to moderate atopic dermatitis, and a recent itch flare-up. A total of 65 subjects participated in the study; 31 subjects used AVEENO® Eczema Therapy Daily Moisturizing Cream (22 female, 9 male) and 34 subjects used the non-fragranced lotion (27 female, 7 male). Overall, 75% of the subjects were female and 25% male, with an average age of 34.5 years. During the course of the study, subjects applied the assigned test material on all areas of the body (including face), as directed. Subjects were also provided with AVEENO® Baby Cleansing Therapy Moisturizing Wash to use in place of normal body wash and/or soap. Subjects used the test materials twice per day for 14 days followed by a 7-day regression period during which treatment was removed. Clinical evaluations were conducted at Baseline, Day 1, 3, 7, 14, and 21. Subjects underwent the following procedures at each of the indicated time points: clinical assessment including Eczema Area Severity Index (EASI) and Atopic Dermatitis Severity Index (ADSI), self-assessment questionnaires, including itch severity (Visual Analog Scale (VAS), digital imaging, non-invasive measures of skin (barrier function TEWL, skin hydration, pH), and microbiome sampling.
TABLE 1. Summary of Demographic Information (ITT Population)
8.9_table_1.jpg
RESULTS
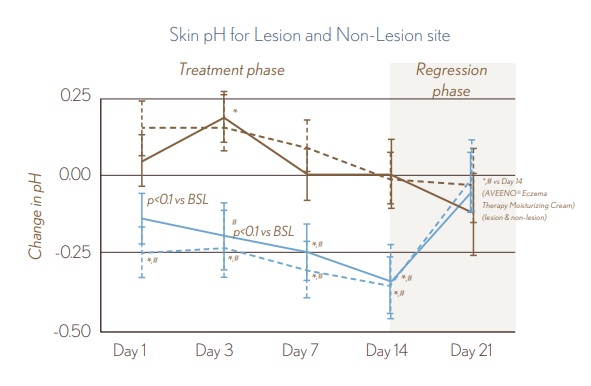
FIGURE 1. Skin Barrier Measurements • Use of AVEENO® Eczema Therapy Daily Moisturizing Cream resulted in a statistically significant decrease in pH from Baseline to Day 14 on lesional and non-lesional skin. Removal of treatment resulted in the pH rebounding. Limited skin pH improvements during treatment phase were seen for the non-fragranced lotion.
8.9_figure_1.jpg
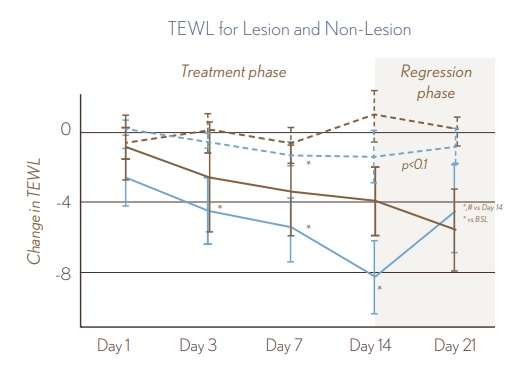
Use of AVEENO® Eczema Therapy Daily Moisturizing Cream resulted in a statistically significant improvement in barrier function, as measured by a decrease in TEWL values for lesional skin at Days 3, 7, and 14. No TEWL improvements during treatment phase were seen for the non-fragranced lotion.
8.9_figure_1b.jpg
Treatment with AVEENO® Eczema Therapy Daily Moisturizing Cream resulted in a statistically significant increase in skin hydration of lesional and non-lesional skin at Days 1, 3, 7, and 14. Treatment with AVEENO® Eczema Therapy Daily Moisturizing Cream demonstrated a significantly greater improvement in skin hydration compared to the non-fragranced lotion (p<0.05).
8.9_figure_1c.jpg
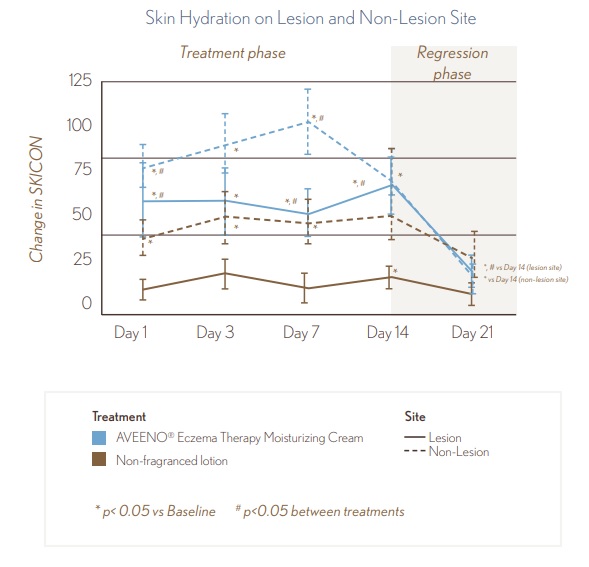
FIGURE 2. ADSI and EASI Clinical Evaluation
- Treatment with AVEENO® Eczema Therapy Daily Moisturizing Cream resulted in ~50% improvement in EASI clinical scoring at Day 14 (p<0.05).
- Treatment with AVEENO® Eczema Therapy Daily Moisturizing Cream resulted in ~50% improvement in ADSI clinical scoring starting at Day 7 (p<0.05).
- Despite the withdrawal of treatment at Day 14, ADSI and EASI clinical scores remained improved from Baseline at Day 21, and were approximately equivalent to Day 3 and Day 7 values, respectively.
8.9_figure_2.jpg
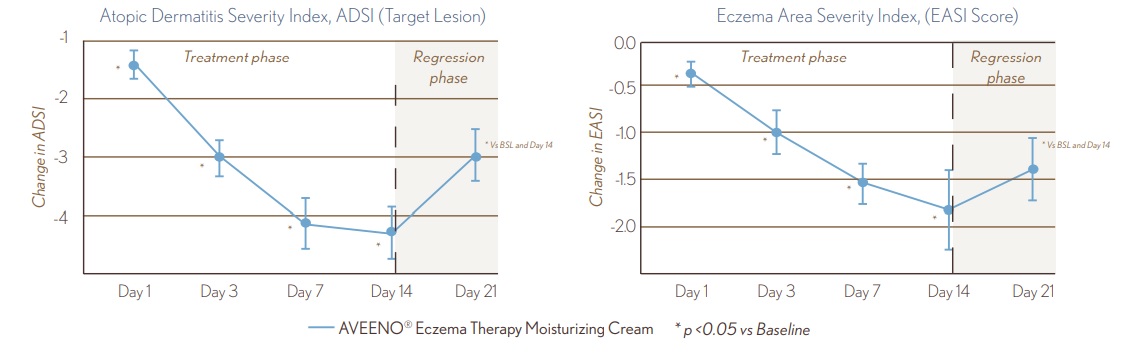
FIGURE 3. VAS Measure of Itch
- Itch Severity improves starting at Day 1 (p<0.05)
- Itch Severity improves starting at Day 1 (p<0.05) and continues throughout treatment, with a 60% improvement after 14 days.
- AVEENO® Eczema Eczema Therapy Daily Moisturizing Cream provided significantly better itch reduction than the non-fragranced lotion (p<0.05) at Day 3
- After 1 week withdrawal of both treatments, itch severity worsened vs. Day 14 but was still improved vs. Baseline (p<0.05).
8.9_figure_3.jpg
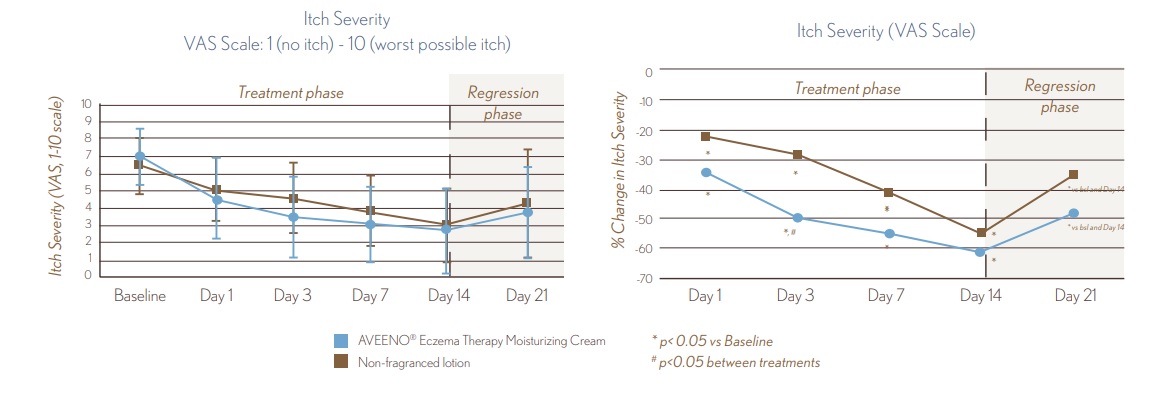
FIGURE 4. Microbial Diversity at Baseline and Changes with Treatment
- At baseline, skin microbial communities of non-lesional skin had significantly greater diversity than lesional skin (P0.05)
- For AVEENO® Eczema Therapy Daily Moisturizing Cream, microbial diversity of lesional skin converged with non-lesional skin starting at Day 1 and remained similar throughout treatment & regression phases P>0.05.
8.9_figure_4.jpg
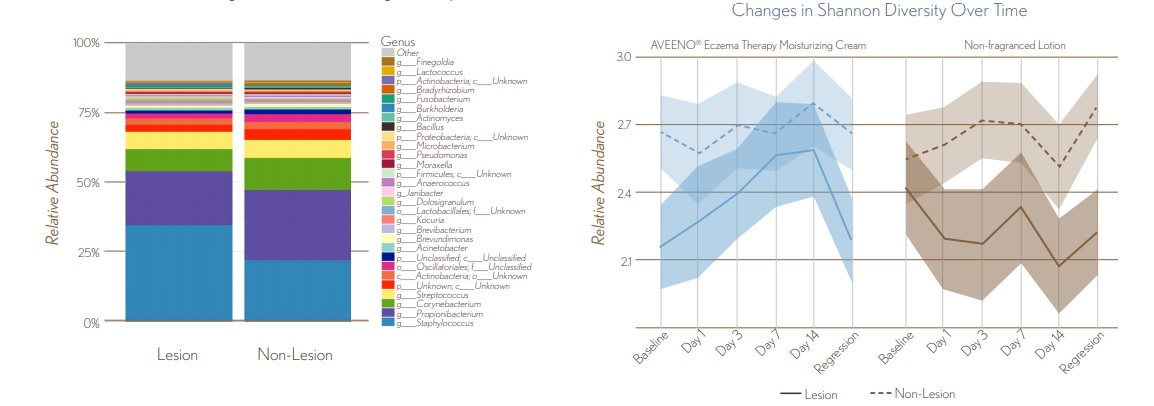
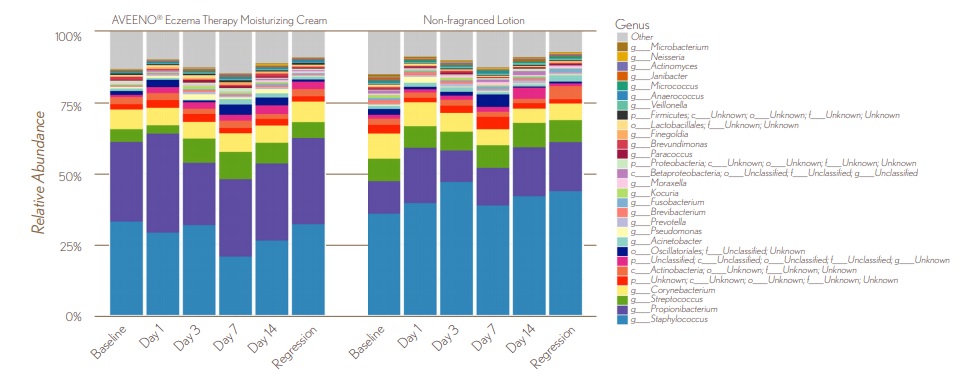
FIGURE 5. Average Differences in Lesional Microbial Diversity
8.9_figure_5.jpg
CONCLUSIONS
At baseline, lesional skin had a higher pH, TEWL and lower skin hydration than non-lesional skin. For subjects treated with AVEENO® Eczema Therapy Daily Moisturizing Cream, disease severity significantly improved from baseline throughout the 14-day treatment. Both lesional and nonlesional skin had significant increases in hydration along with reduced pH, TEWL and itch, in contrast to minimal improvements following use of non-fragranced lotion. At baseline, skin microbial communities of non-lesional skin had significantly greater diversity than lesional skin. Throughout the 14-day treatment with AVEENO® Eczema Therapy Daily Moisturizing Cream there were significant improvements in microbial diversity of lesional skin, with lesional skin microbial diversity reaching that of non-lesional skin by Day 1 and remaining similar throughout treatment and regression phases. These data confirm previous linkages between microbial diversity and AD severity, while extending current knowledge of various microbial populations present in AD lesional skin, which may aid in development of new microbiome-based therapies.
ACKNOWLEDGEMENTS
The authors would like to thank Jeremy Wilkinson at RTL Genomics, for skin microbiome sequencing and data analyses assistance.


