Clinical trial to determine the benefit of AVEENO® Eczema Therapy Itch Relief Balm on dry, itchy skin of adults and children with atopic dermatitis1
Objective:
To evaluate the efficacy of an over-the-counter (OTC) 1% colloidal oatmeal skin protectant balm in adults and children with mild-to-moderate atopic dermatitis (AD).
Study Design:
Fifty-two adults and children aged 12+ years with mild to moderate atopic dermatitis (score 3.0 and 7.5 inclusive per Rajka-Langeland severity index) and mild-to-moderate itch (VAS Itch Assessment = 4.0) completed this multi-center, 7-day, randomized (3:1), evaluator-blinded, 2-arm clinical study.
Efficacy Measures:
Primary endpoints: Itch Assessment (10-cm VAS scale) at baseline, immediately post-application, Hours 1, 2, 3, 4, 5, and 6 post-application, and Day 7
Secondary endpoints: Corneometer readings at baseline, immediately post-application, Hours 1, 2, 3, 4, 5, and 6 post-application, and Day 7
Participant Questionnaires at baseline, immediately post-application, Hours 4, 5, and 6 post-application, and Day 7
eczema_balm_clinical_study_image.jpg
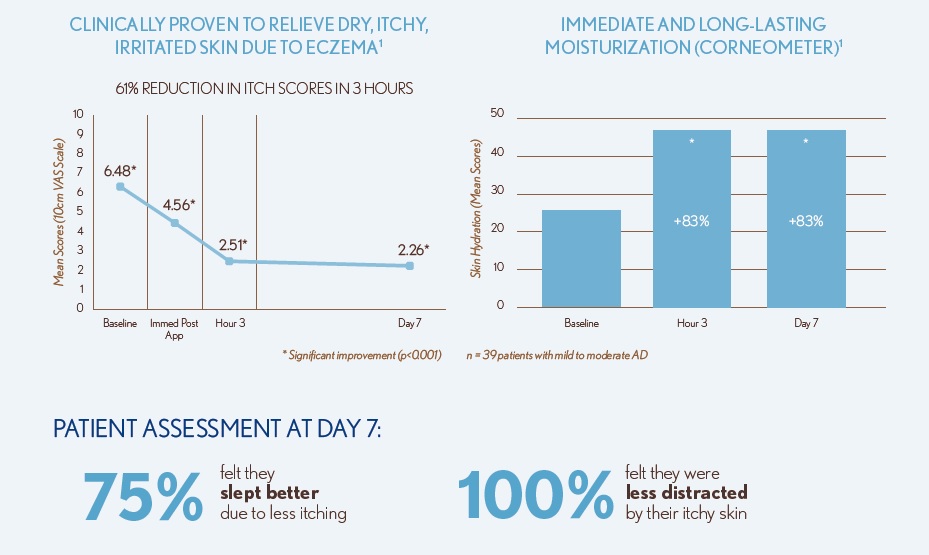
- Data on file. n=39 patients aged 12+ with mild to moderate atopic dermatitis.